1.3: Tertiary Treatment
- Page ID
- 5711
Learning Outcomes
- Explain which water quality characteristics tertiary treatment is targeted to remove
- Describe the breakpoint chlorination curve
- Understand how the mixture of ammonia and chlorine creates chloramines
- Compare different methods of wastewater disinfection
Filtration
After the wastewater leaves the secondary clarifiers, filtration is commonly used to remove fine particles that were carried over in the clarifier. Conventional filtration methods use sand and anthracite coal to filter the treated wastewater. The sand and anthracite coal are referred to as media. The filtration process works by gravity. If needed, the water will be pumped to a higher elevation, then the gravity will force the water through the media. The media creates small voids between the grains of sand and coal. These voids are small enough that water molecules will pass through but the solids will be trapped. Underneath the media, there is an underdrain system that will collect the treated water and convey it to the next treatment process. Eventually, the media will be clogged with solids and needed to be backwashed. During a backwash cycle, the filter is isolated so water is entering the filter. Air is then piped into the filter that agitates the media and separates the solids from the media. The air is then turned off and water is pumped from the bottom of the tank and is directed towards a backwash drain. The media is typically heavier than the solids being removed so the media will settle back to the bottom of the filter while the solids are carried with the water into the backwash drain. When the backwash cycle is complete the filter is put back online.
More advanced filtration methods include membrane filtration such as microfiltration and reverse osmosis. These filtration methods work in a similar manner as sand filters but are able to filter smaller sizes. The membranes are manufactured and can have very small pores. Membrane filtration is able to remove contaminants such as arsenic, asbestos, atrazine, fluoride, lead, mercury, nitrate, radium, benzene, and other unwanted chemicals. With the very small micro-holes in the membrane, gravity does not provide enough pressure to force the water through the membrane. Therefore, the water is pumped through the membrane filtration system which increases the pressure.
Disinfection
Disinfection is a critical treatment step to ensure that the wastewater treatment facility is protecting public health and the environment. Pathogenic organisms, such as E. coli, Vibrio cholerae, and Salmonella, can spread disease to aquatic life and humans. There are other bacteria, microorganisms, and viruses that can be present in wastewater. The disinfection process will limit the presence of pathogens. It’s important to note that the disinfection process is not the same as sterilization. Sterilization will remove all of the bacteria but comes at an extremely high cost that is uneconomical considering the volume of wastewater that must be treated. However, disinfection is still highly effective at limiting pathogens to a level that is acceptable to protect public health and the environment.
Chlorination and Chloramination
Chlorine is the most common form of disinfectant in the United States and has been in use for over one hundred years in the water and wastewater industry. Chlorine is commercially available as gaseous chlorine or in the liquid form as sodium hypochlorite. Gaseous chlorine is purer, so less gas is needed for disinfection. However, because of the high purity, gaseous chlorine can be very dangerous to work around. Treatment facilities that use gaseous chlorine have to comply with rigorous safety training and purchasing regulations. Sodium hypochlorite is commonly sold with a chlorine concentration of 12.5%. Safety precautions must still be used when handling sodium hypochlorite but is a lot safer and easier to work with than gaseous chlorine.
Chlorination works due to the fact that chlorine is highly reactive. When chlorine is added to the wastewater it begins to react with all of the chemicals and organic matter in the wastewater. This is known as demand. Once the chlorine demand is met the extra chlorine added will begin to react with the water creating hypochlorous and hydrochloric acid. Both of these chemicals will be created and the amount of each will depend on the pH of the water.
Hypochlorous acid is more efficient at disinfection and is more prominent at lower pH values. Typical wastewater has a pH of around 6.5 - 7.5 and hypochlorous is the predominant acid. If the pH is above 7.5, treatment facilities may look into adding chemicals to reduce the pH to increase the efficacy of disinfection. When operating chlorination in this manner it’s called free chlorine. Enough chlorine has been added to satisfy the demand and the residual is measured as free chlorine. Free chlorine is highly reactive and is a strong disinfectant. Because it’s highly reactive, the residual does not persist for a long time.
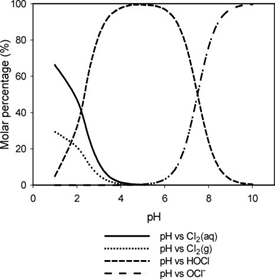
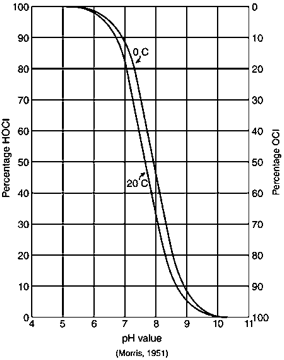
An alternative to chlorination is chloramination. Chloramination is a combined chlorine compound and is created by mixing chlorine and ammonia. Depending on the ratio of chlorine to ammonia, the chloramines created are monochloramine, dichloramine, and trichloramine. Monochloramine is the desired form as it provides a better disinfectant. A ratio of five parts chlorine to one part ammonia will typically yield monochloramine. Chloramination provides some benefits to chlorination. Less chlorine is used since all of the demand doesn’t have to be met. Free chlorine has the potential of creating disinfection-by-products. Since chloramination is not as reactive, there is less chance of creating disinfection-by-products. One disadvantage of chloramination is that, since it’s not as reactive as free chlorine, it takes a longer time to achieve the same level of disinfection as free chlorine.
The relationship between the different chloramines and free chlorine is best understood by examining the breakpoint chlorination curve.
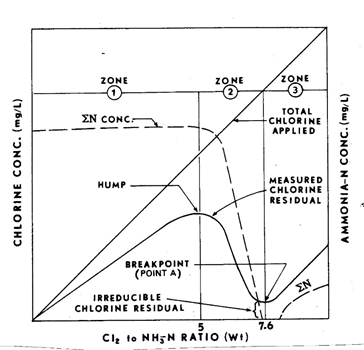
In the first part of the breakpoint curve, chlorine is applied but no residual is seen. This is because there is an excess demand for organic matter and other chemicals. Once that initial demand has been met, more chlorine is applied and a combined residual is formed. In this second zone, ammonia begins to react with the chlorine to create chloramines. In this early stage, monochloramine is the predominant form. If the treatment plant is utilizing chloramination, then ammonia will be added to purposefully stay in this zone. As more chlorine is applied, the ratio of chlorine to ammonia increases. In the third zone, the chlorine residual will decrease as the extra chlorine further reacts with the ammonia creating dichloramine and trichloramine. Once the chlorine has reacted with all of the ammonia, it reaches a breakpoint. After the breakpoint, only free chlorine is available.
Dechlorination
Once the wastewater has been properly disinfected the chlorine residual must be neutralized. Chlorine can be harmful to aquatic life and the environment. Since most wastewater is discharged into a waterbody it must be dechlorinated. There are two common chemicals used for dechlorination, sulfur dioxide, and sodium bisulfite. Sulfur dioxide is available in its gaseous form and has a lot of the same equipment and safety regulations requirements as gaseous chlorine. Therefore, if a treatment plant uses gaseous chlorine for disinfection, it will most likely be using sulfur dioxide for dechlorination. Sodium bisulfite is available in its liquid form is commonly used at treatment facilities that use sodium hypochlorite for disinfection.
UV disinfection
Chlorine is not the only method that can be used for disinfecting pathogens in wastewater. Ultraviolet (UV) light is becoming a popular alternative. UV disinfection is a physical process rather than a chemical process, like chlorination and chloramination. Because no chemicals are used, there is no residual effect that could be harmful to public health and the environment. UV disinfection works by the intensity of the UV light that disrupts the cell walls of the pathogens. Because the light needs to come in contact with the bacteria, it is critical that the turbidity of the water being disinfected is low. If there is too much turbidity in the wastewater, the pathogens will be shielded by the material causing the high turbidity.
Ozonation
Ozonation is more widely used in Europe and Asia. Ozone is three oxygen molecules bonded together, O3. Ozone is highly reactive and must be generated onsite and directly mixed in with the wastewater being disinfected. Ozone is made by taking atmospheric oxygen, O2, and using electricity to break apart the bond between the two oxygen molecules. The individual oxygen molecules then combine with existing O2 molecules to create O3. When the O3 reacts with the wastewater it will create hydrogen peroxide (H2O2) and hydroxyl (OH). These compounds are highly reactive and will disinfect the pathogenic organisms in the wastewater.


