Chapter 2: Introduction to Chemistry and Matter
- Page ID
- 38883
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After reading this section, you should be able to:
- Discuss matter, elements, molecules, and atoms
- Explain electronegativity
- Explain chemical bonding and compounds
- Explain chemical equations and reactions
- Discuss physical states of matter
This chapter presents the fundamentals of chemistry, starting with an introduction on matter and its elemental constituents. Molecular arrangements and chemical bonding are then introduced, followed by examples of chemical nomenclature.
Composition of Matter
Matter can be viewed as anything that has a mass and occupies a space, i.e., that has a specific volume. Mass is defined as a measurement of the quantity of matter present.
|
|
Pin It! Misconception Alert Mass is different than weight though people commonly use them interchangeably. Mass is how much matter is present. Weight is a measure of the gravitational pull on the object. So, your weight will change if you travel to the moon, but your mass will not! |
Elements and Atoms
At the center of all matter are elements. Elements are basic substances that cannot be broken down without altering their basic identities; they cannot be further simplified (e.g., hydrogen, H; oxygen, O). An atom is the smallest amount of an element. The center of each atom contains a nucleus made of protons (very small particles with a positive electric charge) and neutrons (very small particles, without electrical charge), with electrons (very small, negatively charged particles) that gravitate around the nucleus. Electrons have an insignificant mass compared to protons and neutrons.

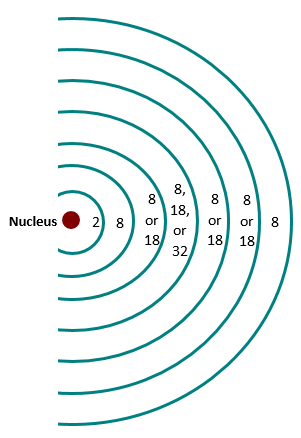
Electrons gravitate around the nucleus of protons and neutrons in layers or shells (Figure \(\PageIndex{1}\). There is only a limited number of electrons per shell, as shown in Figure \(\PageIndex{2}\.
Atoms have the same number of protons and electrons; thus, atoms have a neutral electrical charge. However, most atoms tend to gain or lose electrons to complete their last electron shell and obtain a stable electron configuration. Only the Noble Gasses (such as helium, neon, and argon) do not tend to gain or lose electrons because their electron configuration is naturally stable. An atom with an unequal number of protons and electrons is called an ion. Atoms that lose electron(s) become positively charged and are called cation (e.g., sodium, Na+). Atoms that gain electron(s) become negatively charged and are called anion (e.g., chloride, Cl-). Note that only electrons are gained, lost, or shared because they are readily available; only radioactive compounds can release protons and neutrons.
|
|
Pin It! Misconception Alert Be careful distinguishing between cations and anions. An atom with an unequal number of protons and electrons is an ion. Cations are positively charged ions and anions are negatively charged ions. |
The Periodic Table illustrates all elements that have been found or were synthesized to date (Figure \(\PageIndex{3}\). In this table, elements are ordered in increasing number of protons (from left to right in each row; rows are called periods) and are grouped in columns (called groups) by electron configuration. For example, chlorine appears as number 17 in the Periodic Table (i.e., its atomic number is 17), which means that it has 17 protons. Chlorine is part of the Halogen Family, which all tend to gain an electron to stabilize their last electron shell. While gaining this electron, they become negative charged (e.g., chlorine becomes chloride, Cl-). Each column represents a family, e.g., Alkali Metals (Column 1), Alkali Earth Metals (Column 2), Halogens (Column 17), and Noble Gases (Column 18). Some families are named after their first element, e.g., Boron Family (Column 13), Nitrogen Family (Column 15), and Oxygen Family (Column 16). Additional characteristics are presented later.

The valence (also called the ionic state or oxidation state) is the number of electrons gained, lost, or shared between atoms. Because all elements of a family share similar electron configuration, they all tend to have the same valence, as follows:
- Noble Gases are stable, thus their valence is 0.
- Alkali Metals tend to lose one electron, thus their valence is +1.
- Alkali Earth Metals tend to lose two electrons, thus their valence is +2.
- Elements of the Boron Family tend to lose three electrons, thus their valence is +3.
- Halogens tend to gain one electron, thus their valence is -1.
- Elements of the Oxygen Family tend to gain two electrons, thus their valence is -2.
- Elements of the Nitrogen Family tend to gain three electrons, thus their valence is -3.
Certain elements have multiple valences with different characteristics based on their valence. For example, ferrous iron, Fe2+, has lost two electrons (it has a valence of +2) and is highly soluble in water. On the other hand, ferric iron, Fe3+ (valence of +3), has lost three electrons and is insoluble in water, i.e., it forms a solid and precipitates. Trivalent chromium (i.e., chromite, also called chromium 3, Cr (III), or Cr3+) has lost three electrons and has a valence of +3. It is an essential element that helps regulate the body’s use of sugar, proteins, and fats. However hexavalent chromium (i.e., chromate, also called chromium 6, Cr (VI), or Cr6+) has lost six electrons (valence of +6) and is toxic to humans.
Electronegativity is the degree of attraction of an element for electrons; it defines an element’s affinity for electrons. Electronegativity determines whether an atom will gain, lose, or share electrons.
Molecules and Compounds
Molecules or compounds result from the combination of two or more atoms that are chemically joined (or bonded); e.g., oxygen in the air, O2; water, H2O. Atoms will tend to combine in such ways to increase their stability and complete their electron configuration. For some molecules, this means that they will obtain a zero net electrical charge.
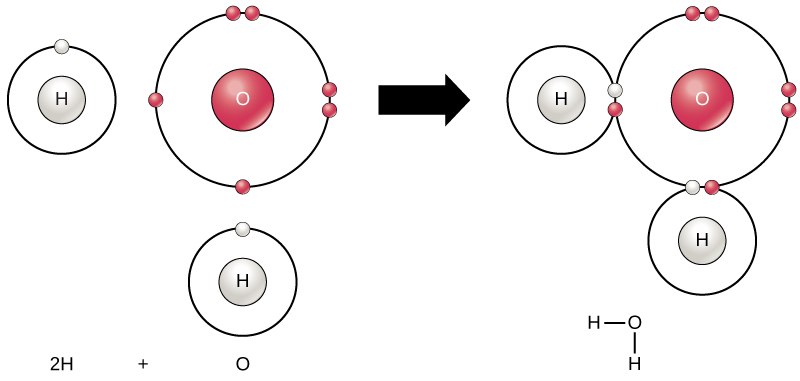
Chemical bonds can be grouped in two broad categories, as illustrated in Figures \(\PageIndex{4}\ and \(\PageIndex{5}\.

- In an ionic bond, electrons are transferred from one atom to another. The atom that loses electron(s) becomes positively charged and is called a cation. Conversely, the atom that gains electron(s) becomes negatively charged and is called an anion. Examples of ionic bonds are shown in Table \(\PageIndex{1}\.
- In a covalent bond, electrons are shared between atoms. The electronegativity of each atom will determine the polarity of the resulting molecule.
Table \(\PageIndex{1}\: Examples of Ionic Bonds
|
Example |
Explanation |
|---|---|
|
Sodium chloride, NaCl |
|
|
Sodium oxide, Na2O |
|
Table \(\PageIndex{2}\: Examples of Covalent Bonds
|
Example |
Explanation |
|---|---|
|
Homonuclear molecules: Equal attraction for the shared electron(s) |
Hydrogen, H2: Single covalent bond: H ̶ H Chlorine, Cl2: Single covalent bond: Cl ̶ Cl Oxygen, O2: Double covalent bond: O = O Nitrogen, N2: Triple covalent bond: N ≡ N |
|
Heteronuclear molecule: Unequal attraction for the shared electron(s) Example: Water, H2O |
Valence of +1: Need 1 bond
Valence of -2: Need 2 bonds
(2 x H+1) + (1 x O2-) = H2O |
The key differences between ionic and covalent bonds are summarized in Table \(\PageIndex{3}\ on the following page.
Table \(\PageIndex{3}\: Comparison of Ionic Bonds and Covalent Bonds
|
Ionic Bonds |
Covalent Bonds |
|---|---|
|
|
Dissociation
Molecules that result from polar covalent bond (i.e., atoms of different types that share electrons) may breakdown or dissociate. This is the case for water, H2O, which dissociates into hydrogen ion, H+, and hydroxide, OH-. The dissociation of water is measured as pH.
Chemical Names1
The Period Table (Figure \(\PageIndex{3}\) presents all the elements that are known to mankind to this day.
Cations keep is the same name as the element name listed in the Periodic Table. For example, the sodium ion Na+. Anions have "-ide" at the end when they are formed from elements. For example, chloride, Cl- is the anion of the element chlorine.
Binary compounds (contain only two elements) are a little more complicated to name based on whether they have ionic or covalent bonds. If they are composed of a metal and a polyatomic ion (non-metal), then you use the name of the metal in the periodic table and then the name of the polyatomic ion. If the polyatomic ion is a single element, then change the ending to “ide”, even if one element has multiple atoms: for example, magnesium chloride, MgCl2.
Metals with variable valences, such as the Transition Metals (Columns 3 through 12 of the Periodic Table, Figure \(\PageIndex{3}\), are more complex because they are followed by a symbol that reflects the valence. This was introduced earlier in Section 2.1.1. For example, trivalent chromium (i.e., chromite) has a valence of +3, and is referred to as Cr(III), or Cr3+; hexavalent chromium (i.e., chromate) has a valence of +6 and is referred to as Cr(VI), or Cr6+.
Naming acids and their derivatives is more complex and may depend on the acid’s oxidation state. Generally, the acids with the highest oxidation state end in –ic; e.g., sulfuric acid (H2SO4), nitric acid (HNO3), or phosphoric acid (H3PO4). Their salts end in –ate; e.g., sulfate (SO42-), nitrate (NO3-), or phosphate (PO42-). Acids with the next lowest oxidation state end in –ous; e.g., sulfurous acid, H2SO3. Their salts end in –ite; e.g., sulfite, SO32-. Acids with the lowest oxidation state begin in hypo– and end in –ous; e.g., hypochlorous acid, HOCl. Their salts begin in hypo– and end in –ite; e.g., hypochlorite ion, OCl-.
Physical States of Matter
States of matter are distinct forms that matter can take. They are based on how elements are arranged in matter. Matter can take four different states (Figure \(\PageIndex{6}\):
- Solid: Volume and shape are fixed;
- Liquid: Fixed volume, but variable shape that adapts to its container;
- Gas: Volume and shape are variable;
- Plasma: Variable volume and shape, with electrical charges.
The physical states of matter reflect different energy levels: solids have the lowest energy level, followed by liquids. Gases have higher energy levels, and plasma have the highest energy level. In water, the first three states (i.e., solid, liquid and gas) are important.

Key Terms
- anion – an atom that gains electrons and becomes negatively charged
- cation – an atom that loses one or more electrons and becomes positively charged
- covalent bond – A type of bond in which electrons are shared between atoms; organic compounds; low melting point; solid, liquid or gas at room temperature; poor conductor; resulting substance is called a molecule or molecular compound
- electronegativity – the degree of attraction of an element for electrons; it defines an element’s affinity for electrons. Electronegativity determines whether an atom will gain, lose, or share electrons.
- ionic bond – Transfer of electron(s) from one atom to another; tend to be inorganic; High melting point; often solid at room temperature; good conductor; resulting substance is called a compound
- mass – a measurement of the quantity of matter present
- matter – anything that has a mass and occupies a space; can take on four states (solid, liquid, gas, and plasma)
- periodic table – an organization of all elements that have been found or synthesized in order from increasing number of protons (from left to right in each row); rows are called periods and columns are called groups
- valence - also called the ionic state or oxidation state; the number of electrons gained, lost, or shared between atoms. Because all elements of a family share similar electron configuration, they all tend to have the same valence
[1] Nomenclature is shared under a CC BY license and was authored, remixed, and/or curated by LibreTexts.


