Chapter 3: Chemical Equations
- Page ID
- 38889
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After reading this section, you should be able to:
- Describe ionic, covalent, and hydrogen bonding
- Explain synthesis, decomposition, exchange, and combustion reactions
- Describe the energy flow through chemical reactions
Bonding1
The model of the atom presented in Chapter 2 is known as the “Bohr” model, and was developed in 1913, by Danish scientist Niels Bohr (1885–1962). His model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular orbitals at specific distances from the nucleus, as Figure 3.1 illustrates. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. These energy levels are designated by a number and the symbol “n.” For example, 1n represents the first energy level located closest to the nucleus.
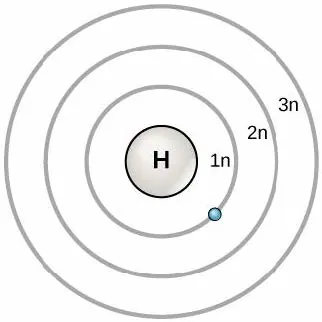
Electrons fill orbitals in a consistent order: they first fill the orbitals closest to the nucleus, then they continue to fill orbitals of increasing energy further from the nucleus. If there are multiple orbitals of equal energy, they fill with one electron in each energy level before adding a second electron. The electrons of the outermost energy level determine the atom's energetic stability and its tendency to form chemical bonds with other atoms to form molecules. We refer to the outermost energy level as the valence shell.
Under standard conditions, atoms fill the inner shells first, often resulting in a variable number of electrons in the outermost shell. The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons. With the exception of the innermost shell, atoms are more stable energetically when they have eight electrons in their valence shell. This is an atom’s natural preferred configuration. As such, an atom may give, take, or share electrons with another atom to achieve a full valence shell, and is how covalent and ionic bonds are formed. Refer back to Tables 2.1 and 2.2 in Chapter 2, for examples of these types of bonds.
Remember,
- In an ionic bond, electrons are transferred from one atom to another. The atom that loses electron(s) becomes positively charged and is called a cation. Conversely, the atom that gains electron(s) becomes negatively charged and is called an anion. Examples of ionic bonds are shown in Table 2.5.
- In a covalent bond, electrons are shared between atoms. The electronegativity of each atom will determine the polarity of the resulting molecule.
There is one more bond to talk about, the hydrogen bond.
Hydrogen Bonds2
A hydrogen bond is a special type of bond which occurs when a hydrogen atom bonded to a strongly electronegative atom (like oxygen) in the vicinity of another electronegative atom with a lone pair of electrons. These bonds are much weaker than covalent and ionic bonds.
For a hydrogen bond to occur there must be both a hydrogen donor and an acceptor present. The donor in a hydrogen bond is the atom to which the hydrogen atom participating in the hydrogen bond is covalently bonded, and is usually a strongly electronegative atom such as N, O, or F. The hydrogen acceptor is the neighboring electronegative ion or molecule and must possess a lone electron pair in order to form a hydrogen bond.
The most common example of hydrogen bonding in the natural world occurs between molecules of water. It happens before your eyes whenever two raindrops merge into a larger bead, or a creek spills into a river. Hydrogen bonding occurs because the weakly negative oxygen atom in one water molecule is attracted to the weakly positive hydrogen atoms of two other water molecules (Figure \(\PageIndex{2}\)).

Water molecules also strongly attract other types of charged molecules as well as ions. This explains why “table salt,” for example, actually is a molecule called a “salt” in chemistry, which consists of equal numbers of positively-charged sodium (Na+) and negatively-charged chloride (Cl–), dissolves so readily in water, in this case forming dipole-ion bonds between the water and the electrically-charged ions (electrolytes). Water molecules also repel molecules with nonpolar covalent bonds, like fats, lipids, and oils. You can demonstrate this with a simple kitchen experiment: pour a teaspoon of vegetable oil, a compound formed by nonpolar covalent bonds, into a glass of water. Instead of instantly dissolving in the water, the oil forms a distinct bead because the polar water molecules repel the nonpolar oil.
Types of Hydrogen Bonds
Hydrogen bonds can occur within one single molecule, between two like molecules, or between two unlike molecules.
- Intramolecular hydrogen bonds: Intramolecular hydrogen bonds are those which occur within one single molecule. This occurs when two functional groups of a molecule can form hydrogen bonds with each other. In order for this to happen, both a hydrogen donor an acceptor must be present within one molecule, and they must be within close proximity of each other in the molecule. For example, intramolecular hydrogen bonding occurs in ethylene glycol (C2H4(OH)2) between its two hydroxyl groups due to the molecular geometry.
- Intermolecular hydrogen bonds: Intermolecular hydrogen bonds occur between separate molecules in a substance. They can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present in positions in which they can interact. For example, intermolecular hydrogen bonds can occur between NH3 molecules alone, between H2O molecules alone, or between NH3 and H2O molecules.
Hydrogen bonding plays a crucial role in many biological processes and can account for many natural phenomena such as the unusual characteristics of water. In addition to being present in water, hydrogen bonding is also important in the water transport system of plants, protein structure, and nucleic acids.
Molecular Mass and Moles3
The molecular mass of a substance is the sum of the average masses of the atoms in one molecule of a substance. It is calculated by adding together the atomic masses of the elements in the substance, each multiplied by its subscript (written or implied) in the molecular formula. Because the units of atomic mass are atomic mass units, the units of molecular mass are also atomic mass units.
A mole is defined as the amount of a substance that contains the number of carbon atoms in exactly 12 g of isotopically pure carbon-12. According to the most recent experimental measurements, this mass of carbon-12 contains 6.022142 × 1023 atoms, but for most purposes 6.022 × 1023 provides an adequate number of significant figures. Just as 1 mole of atoms contains 6.022 × 1023 atoms, 1 mole of eggs contains 6.022 × 1023 eggs. The number in a mole is called Avogadro’s number, after the 19th-century Italian scientist who first proposed how to measure the number of molecules in a gas.
The mole provides a bridge between the atomic world (amu) and the laboratory (grams). It allows determination of the number of molecules or atoms by weighing them. The numerical value of Avogadro's number, usually written as No, is a consequence of the arbitrary value of one kilogram, called the International Prototype Kilogram, and the choice of reference for the atomic mass unit scale, one atom of carbon-12. Why? Because the number of carbon atoms in exactly 12 g of isotopically pure carbon-12 contains almost exactly 6.022 x 1023 atoms.
So how much does 1 mole of H2O weigh? The atomic mass of hydrogen is 1. We have two of them in water. The atomic mass of oxygen is 16. So (2 x 1)H + (16)O = 18 g
Chemical Reactions4
Chemical energy is the form of potential energy in which energy is stored in chemical bonds. When those bonds are formed, chemical energy is invested, and when they break, chemical energy is released. Notice that chemical energy, like all energy, is neither created nor destroyed; rather, it is converted from one form to another.
All chemical reactions begin with a reactant, the general term for the one or more substances that enter into the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. The one or more substances produced by a chemical reaction are called the product.
In chemical reactions, the components of the reactants—the elements involved and the number of atoms of each—are all present in the product(s). Similarly, there is nothing present in the products that are not present in the reactants. This is because chemical reactions are governed by the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Just as you can express mathematical calculations in equations such as 2 + 7 = 9, you can use chemical equations to show how reactants become products. As in math, chemical equations proceed from left to right, but instead of an equal sign, they employ an arrow or arrows indicating the direction in which the chemical reaction proceeds. For example, the chemical reaction in which one atom of nitrogen and three atoms of hydrogen produce ammonia would be written as N+3H→NH3. Correspondingly, the breakdown of ammonia into its components would be written as NH3→N + 3H.
Notice that, in the first example, a nitrogen (N) atom and three hydrogen (H) atoms bond to form a compound. This anabolic reaction requires energy, which is then stored within the compound’s bonds. Such reactions are referred to as synthesis reactions. A synthesis reaction is a chemical reaction that results in the synthesis (joining) of components that were formerly separate (Figure \(\PageIndex{3}\). Again, nitrogen and hydrogen are reactants in a synthesis reaction that yields ammonia as the product. The general equation for a synthesis reaction is A + B→AB.

In the second example, ammonia is catabolized into its smaller components, and the potential energy that had been stored in its bonds is released. Such reactions are referred to as decomposition reactions. A decomposition reaction is a chemical reaction that breaks down or “de-composes” something larger into its constituent parts (see Figure 3.3). The general equation for a decomposition reaction is: AB→A+BAB→A+B.
An exchange reaction is a chemical reaction in which both synthesis and decomposition occur, chemical bonds are both formed and broken, and chemical energy is absorbed, stored, and released (see Figure \(\PageIndex{3}\)). The simplest form of an exchange reaction might be: A+BC→AB+C. Notice that, to produce these products, B and C had to break apart in a decomposition reaction, whereas A and B had to bond in a synthesis reaction. A more complex exchange reaction might be: AB+CD→AC+BD. Another example might be: AB+CD→AD+BC.
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesize into a product that is later decomposed. Reversibility is also a quality of exchange reactions. For instance, A+BC→AB+C could then reverse to AB+C→A+BC. This reversibility of a chemical reaction is indicated with a double arrow: A+BC⇄AB+C. Many chemical reactions do proceed in a predictable direction, either one way or the other. You can think of this more predictable path as the path of least resistance because, typically, the alternate direction requires more energy.
Combustion Reactions
Combustion reactions are types of chemical reactions where compounds and oxidants react to produce heat and new products. A common combustion reaction is the reaction between oxygen and hydrocarbons to yield water and carbon dioxide:
(Oxygen) + (Hydrocarbon) (Carbon Dioxide) + (Water) + heat
For example:
- 2H2 + O2 → 2H2O + heat
- CH4 + 2O2 → CO2 + 2H2O + heat
It is also common for a combustion reaction to release light and produce a flame. However, it is not necessary. For a combustion reaction to be initiated, the activation energy for the reaction must be overcome. Often combustion reactions are started with a flame, which provides the heat to initiate the reaction. Once combustion begins, the heat that is produced sustains the reaction until the reactants are used.
In order to recognize combustion reactions, oxygen will be on the reactant side of the equation and the release of heat will be on the product side of the equation. Sometimes the fuel molecule contains oxygen. For example, the combustion of ethanol:
C2H5OH + 3O2 → 2CO2 + 3H2O
Combustion is an exothermic reaction so that it releases heat. However, sometimes the reaction proceeds so slowly that a temperature change is not noticeable. The signs that a combustion reaction occurs is the presence of oxygen as a reactant and carbon dioxide, water, and heat as products. Inorganic combustion reaction may not form the same types of products; however, they are recognizable by the reaction of oxygen. The surest method of recognizing a combustion reaction is that the products contain carbon dioxide and water.
Combustion does not always proceed to completion or 100-percent efficiency. The reactions are prone to limiting reactants. Two types of combustion reactions exist:
- Complete Combustion: known as clean combustion where oxidation of the reactants (hydrocarbons) produces only carbon dioxide and water. The burning of candle wax, where the heat from the wick vaporizes the wax (hydrocarbon), is an example. The reaction results from the oxygen in the air so that carbon dioxide and water are the products. All of the wax burns so that nothing remains once the candle is consumed. The water vapor and carbon dioxide dissipate into the atmosphere.
- Incomplete Combustion: incomplete combustion is the oxidation of a hydrocarbon that is incomplete or dirty. The products are carbon monoxide and carbon (soot) in addition to carbon dioxide. An example would be the combustion of coal, where soot and carbon monoxide are the products. Fossil fuels oxidize incompletely, and they release waste products.
Energy Flow in Chemical Reactions
Chemical bonds represent stored chemical energy, and chemical reactions ultimately result in net absorption or release of energy. Reactions that release energy are called exergonic reactions. These reactions yield products with less energy than the initial reactants, along with energy that can be harvested for use. Catabolic and oxidative reactions are exergonic for the most part.
The products of energy absorbing, endergonic, reactions contain potential energy in their chemical bonds which is more than the energy that the reactants contained. Anabolic reactions are energy absorbing reactions. For example, the energy released when fuel molecules are broken down (oxidized) is captured in ATP molecules and used to synthesize complex biological molecules the body needs to sustain life.
Factors that Influence the Rate of Chemical Reactions6
If chemical reactions are to occur quickly, the atoms in the reactants have to have easy access to one another. Thus, the greater the surface area of the reactants, the more readily they will interact. As a general rule, gases tend to react faster than liquids or solids, again because it takes energy to separate particles of a substance, and gases by definition already have space between their particles. Similarly, the larger the molecule, the greater the number of total bonds, so reactions involving smaller molecules, with fewer total bonds, would be expected to proceed faster.
Reactions that involve highly reactive elements like hydrogen proceed more quickly than reactions that involve less reactive elements. Reactions involving stable elements like helium are not likely to happen at all.
Increasing the temperature of a substance increases the kinetic energy of its particles and the force of their collisions. Chemical reactions proceed more quickly at higher temperatures.
Chemical reactions progress more rapidly when the reacting particles are present in high numbers, concentrations, because the chance of collisions are greater. As the concentrations of the reactants declines, the reaction slows. Chemical equilibrium eventually occurs unless additional reactants are added or products are removed from the reaction site.
Smaller particles move faster than large particles and tend to collide more frequently and more forcefully. The smaller the reacting particles, the faster a chemical reaction proceeds at a given temperature and concentration.
Chemical reactions in nonliving systems can be speeded up by heating. However drastic increases in body temperature are life threatening because important biological molecules are destroyed. At normal body temperature, most chemical reactions would proceed at too slow of a pace to maintain life, if catalysts were not present. Catalysts are substances that increase the rate of chemical reactions without becoming chemically changed or part of the product. Biological catalysts are called enzymes.
Key Terms
catalysts – substances that increase the rate of chemical reactions without becoming chemically charged or part of the product; biological catalysts are called enzymes
combustion reactions – types of chemical reactions where compounds and oxidants react to produce heat and new products
decomposition reactions – a reaction in which larger molecules are split into smaller molecules, ions, or atoms
mole – one mole of a substance is its molecular weight expressed in grams
synthesis reactions – a reaction in which two or more atoms, ions, or molecules combine to form new and larger molecules
[1] 2.1 Atoms, Isotopes, Ions, and Molecules: The Building Blocks by OpenStax is licensed under CC BY 4.0 and was modified by Donald Kendall.
[2] Hydrogen Bonding is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Jose Pietri.
[3] This page titled Chapter 1.7: The Mole and Molar Mass is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous.
[4] 2.3 Chemical Reactions by OpenStax is licensed and modified under CC BY 4.0.
[5] Image by OpenStax is licensed under CC BY 4.0.
[6] 2.3 Chemical Reactions by OpenStax is licensed and modified under CC BY 4.0.

