Chapter 8: Total Coliform Rule
- Page ID
- 38916
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After reading this section, you should be able to:
- Describe biofilms
- Explain common microbiological techniques
- Outline and describe coliform bacterial analysis
Biofilms
Bacteria may grow and persist in the environment as free floating (planktonic) cells in liquids or attached to surfaces as biofilm. In 1683, Antoni van Leeuwenhoek observed and described biofilms by using his primitive microscope on matter from his own teeth. Biofilm form when bacteria adhere to environmental surfaces, especially those located in the presence of high moisture. Biofilm typically persist in a matrix containing slimy, glue-like substances (extracellular polymeric substances) which facilitate their attachment to many hard surfaces. The biofilm matrix provides embedded bacteria with protection from dehydration and other environmental stresses and may interfere with the action of chemical disinfectants.1

A biofilm is considered to a hydrogel. According to Wang et. al (2020), hydrogels are “three-dimensional (3D) cross-linked polymer networks that can absorb and retain large amount of water. Because of their tunable properties as well as their versatile fabrication methods, hydrogel materials have been applied in a wide range of biomedical and engineering applications, ranging from tissue engineering and regenerative medicine to wastewater treatment…”2
Culture Media
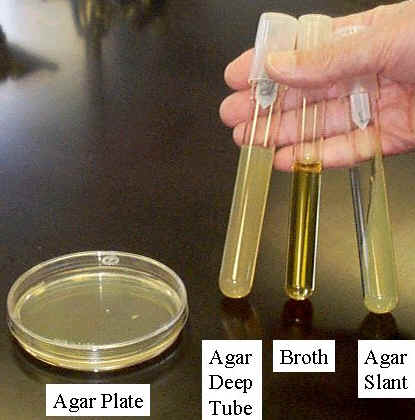
Culture medium or growth medium is a liquid or gel designed to support the growth of microorganisms. There are different types of media suitable for growing different types of cells. Nutrient broths and agar plates are the most common growth media, although specialized media are sometimes required for microorganism and cell culture growth. Some organisms, termed fastidious organisms, need specialized environments due to complex nutritional requirements. Viruses, for example, are obligate intracellular parasites and require a growth medium containing living cells. Many human microbial pathogens also require the use of human cells or cell lysates to grow on a media. The most common growth media nutrient broths (liquid nutrient medium) or LB medium (Lysogeny Broth) are liquid. These are often mixed with agar and poured into Petri dishes to solidify. These agar plates provide a solid medium on which microbes may be cultured. They remain solid, as very few bacteria are able to decompose agar. Many microbes can also be grown in liquid cultures comprised of liquid nutrient media without agar.
|
|
|
|
| Figure \(\PageIndex{2}\): Petri Dish #1. (Copyright; Image by Ninjatacoshell is licensed under CC BY-SA 3.0) | Figure \(\PageIndex{3}\): Petri Dish #2. (Copyright; Image by NASA is in the public domain) | Figure \(\PageIndex{4}\): Petri Dish #3. (Copyright; Image by the CDC is in the public domain) |
Chemically Defined Medium
A defined culture medium will have known quantities of all ingredients. For microorganisms, it provides trace elements and vitamins required by the microbe and especially a defined carbon and nitrogen source. Glucose or glycerol are often used as carbon sources, and ammonium salts or nitrates as inorganic nitrogen sources. A chemically defined medium is entirely free of animal-derived components (including microbial derived components such as yeast extract) and represents the purest and most consistent cell culture environment.
Complex Undefined Media
An undefined medium has some complex ingredients, such as yeast extract or casein hydrolysate, which consist of a mixture of many, many chemical species in unknown proportions. Undefined media are sometimes chosen based on price and sometimes by necessity – some microorganisms have never been cultured on defined media. A defined medium (also known as chemically defined medium or synthetic medium) is a medium in which all the chemicals used are known, no yeast, animal, or plant tissue is present.
Anaerobic Growth Media and Methods
Quite a few human pathogens are strict anaerobes. One way to culture and grow anaerobes is the use of reduced media--media without oxygen. Thioglycollate broth has a reducing agent in it---the chemical thioglycollate---which binds any free oxygen within the medium. These tubes have screw caps, allowing a tight closure, to reduce oxygen entry. However, some oxygen will be in the tube between the cap and the broth and there is no way to get rid of it. So, there will be some diffusion of oxygen into the top portion of the broth, and that is where any aerobic bacteria may grow.
Special Culture Techniques
Microbes are incredibly varied in what they use as a food source, the environments they live in, and the danger levels they may have for humans and other organisms they may compete with. Therefore, they need special nutrient and growth environments that are not easily accommodated. For example, a microaerophile is a microorganism that requires oxygen to survive but requires environments containing lower levels of oxygen than are present in the atmosphere (~20% concentration). Many microphiles also require an elevated concentration of carbon dioxide. In the laboratory they can be easily cultivated in a candle jar. A candle jar is a container into which a lit candle is introduced before sealing the container’s airtight lid. The candle’s flame burns until extinguished by oxygen deprivation, which creates a carbon dioxide-rich, oxygen-poor atmosphere in the jar.
Pure Cultures
It is often essential to isolate a pure culture of microorganisms. A pure (or axenic) culture is a population of cells or multicellular organisms growing in the absence of other species or types. A pure culture may originate from a single cell or single organism, in which case the cells are genetic clones of one another. Microbiological cultures can be grown in petri dishes of differing sizes that have a thin layer of agar-based growth medium. Once the growth medium in the petri dish is inoculated with the desired bacteria, the plates are incubated at the best temperature for the growing of the selected bacteria.
But most bacteria samples contain a variety of different kinds and would be termed a mixed culture. However, it is possible to isolate specific bacteria by depositing the sample into a petri dish with agar with an inoculating loop or sterile cotton swab. using the streak plate method. If you swab a door handle, where bacteria are likely to be present, and then pass the swab across the surface of a petri plate, cells would be deposited onto the surface from the swab. Initially, the swab may have a fairly high concentration of cells and the area touched by it will have lots of different cell types placed close together. After these grow up, the cells’ progeny will crowd together and overlap with other cells’ progeny forming areas called confluent growth. As the swab moves across the agar leaving cells on the agar in a zig zag pattern as shown in Figure 8.6, regions touched later in the process will have fewer and fewer cells. Individual cells are far enough apart that each one would grow into a discrete isolated colony.

)
Coliform Bacterial Analysis
Municipal sewage contains human feces and water contaminated with these effluents may contain pathogenic (disease-causing) organisms and, consequently, may be hazardous to human health if used as drinking-water or in food preparation. Fecal contamination of water is routinely detected by microbiological analysis.
It is impractical to attempt the routine isolation of pathogens because they are present in relatively small numbers compared with other types of microorganism. Moreover, many types of pathogens exist, and each organism requires a unique microbiological isolation technique. The approach that has been adopted is to analyze samples for indicator organisms that inhabit the gut and are excreted in human feces. The presence of these indicator organisms in water is evidence that fecal contamination is present, and a risk exists that pathogens are present.
If indicator organisms are present in large numbers, the contamination is considered to be recent and/or severe. Bacteria in water are, in general, not present individually, but as clumps or in association with particulate matter. When enumerating bacteria in water it is not the number of individual bacteria present which are counted, but the number of clumps of bacteria or the particles and their associated bacteria. Each clump or particle may have many bacteria associated with it.
Total Coliforms
The term total coliforms refer to a large group of Gram-negative, rod-shaped bacteria that share several characteristics. The group includes heat-tolerant coliforms and bacteria of fecal origin, as well as some bacteria that may be isolated from environmental sources. The presence of total coliforms may or may not indicate fecal contamination. In extreme cases, a high count for the total coliform group may be associated with a low, or zero, count for thermos-tolerant coliforms. A result of this nature would not necessarily indicate the presence of fecal contamination. It might be caused by entry of soil or organic matter into the water or by conditions suitable for the growth of other types of coliform. In the laboratory total coliforms are grown in or on a medium containing lactose, at a temperature of 35 or 37 °C. They are provisionally identified by the production of acid and gas from the fermentation of lactose.
The term fecal coliform has been used in water microbiology to denote coliform organisms which grow at 44 or 44.5 C and ferment lactose to produce acid and gas. In practice, some organisms with these characteristics may not be of fecal origin and the term thermos-tolerant coliform is, therefore, more correct and is becoming more commonly used. Nevertheless, the presence of thermos-tolerant coliforms nearly always indicates fecal contamination.
Usually, more than 95 percent of thermos-tolerant coliforms isolated from water are the gut organism Escherichia coli, the presence of which is definitive proof of fecal contamination. As a result, it is often unnecessary to undertake further testing to confirm the specific presence of E. coli.
The presence of fecal streptococci is evidence of fecal contamination. Fecal streptococci tend to persist longer in the environment than thermos-tolerant or total coliforms and are highly resistant to drying. It is, therefore, possible to isolate fecal streptococci from water that contains few or no thermos-tolerant coliforms as, for example, when the source of contamination is distant in time or space from the sampling point. Fecal streptococci grow in or on a medium containing sodium azide, at a temperature of 37-44°C. They are usually detected by the reduction of a dye (a tetrazolium-containing compound) or the hydrolysis of aesculin. Routine methods may give false positives and additional confirmatory tests may be required.
Key Terms
- biofilm – three-dimensional structures that create a matrix referred to commonly as a slime
- culture – the microbes that grow or multiply in or on a culture medium
- culture medium – a nutrient material prepared for the growth of microorganisms in a laboratory
- inoculum – microbes introduced into a culture medium to initiate growth
- streak plate method – an isolation method to achieve pure cultures by streaking a pattern over a surface of nutrient medium
- total coliform – a large group of Gram-negative, rod-shaped bacteria that share several characteristic and include heat-tolerant coliforms and bacteria of fecal origin, as well as some bacteria that may be isolated from environmental sources
[1] (Efficacy Test Methods, Test Criteria, and Labeling Guidance for Antimicrobial Products with Claims Against Biofilm on Hard, Non-Porous Surfaces https://www.epa.gov/pesticide-analyt...eling-guidance)
[2] Wang, W., Narain, R., & Zeng, H. (2020). Hydrogels. Polymer Science and Nanotechnology, 203–244. https://doi.org/10.1016/b978-0-12-816806-6.00010-8




