5.5: Technological Hazards
- Page ID
- 3947
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hazardous materials
Hazardous materials (also known as hazmat) are regulated by a number of federal agencies including the US Department of Transportation, US Environmental Protection Agency, US Nuclear Regulatory Commission, and the Occupational Safety and Health Administration of the US Department of Labor. In addition, the US Coast Guard and Federal Emergency Management Agency of the US Department of Homeland Security have responsibilities for emergency response to hazmat incidents. Because these agencies have different responsibilities, they have correspondingly different definitions of hazmat. According to the Department of Transportation, hazmat is defined as substances that are “capable of posing unreasonable risk to health, safety, and property” (49CFR 171.8).
Until the late 1980s, the location, identity, and quantity of hazmat throughout the United States was generally undocumented. However, Title III of the Superfund Amendments and Reauthorization Act—SARA Title III (also known as the Emergency Planning and Community Right to Know Act—EPCRA) of 1986 required those who produce, handle, or store amounts exceeding statutory threshold planning quantities of approximately 400 Extremely Hazardous Substances (EHSs) to notify local agencies, their State Emergency Response Commission (SERC), and the US EPA. Nonetheless, the Chemical Abstract Service (CAS) lists 1.5 million chemical formulations with 63,000 of them hazardous. There are over 600,000 shipments of hazmat per day (100,000 of which are shipments of petroleum products). Fortunately, only a small proportion of these chemicals account for most of the number of shipments and the volume of materials shipped (see Table 5-7, adapted from Lindell & Perry, 1997a). These hazmat shipments result in an average of 280 liquid spills or gaseous releases per year, the vast majority of which occur in transport. Of these spills and releases, 81% take place on the highway and 15% are in rail transportation. These incidents cause approximately 11 deaths and 311 injuries per year.
Table 5-7. Volume of production for top 12 EHSs, 1970–1994.
|
Rank in top 50 |
Chemical name |
Year |
% increase 1970-1994 |
|||
|
1970 |
1980 |
1990 |
1994 |
|||
|
1 |
Sulfuric acid |
29,525 |
44,157 |
44,337 |
44,599 |
51 |
|
8 |
Ammonia |
13,824 |
19,653 |
17,003 |
17,965 |
30 |
|
10 |
Chlorine |
9,764 |
11,421 |
11,809 |
12,098 |
24 |
|
13 |
Nitric acid |
7,603 |
9,232 |
7,931 |
8,824 |
16 |
|
23 |
Formaldehyde |
2,214 |
2,778 |
3,360 |
4,277 |
93 |
|
25 |
Ethylene oxide |
1,933 |
2,810 |
2,678 |
3,391 |
75 |
|
31 |
Phenol |
854 |
1,284 |
1,769 |
2,026 |
137 |
|
33 |
Butadiene |
1,551 |
1,400 |
1,544 |
1,713 |
10 |
|
34 |
Propylene oxide |
590 |
884 |
1,483 |
1,888 |
220 |
|
36 |
Acrylonitrile |
520 |
915 |
1,338 |
1,543 |
197 |
|
37 |
Vinyl acetate |
402 |
961 |
1,330 |
1,509 |
275 |
|
47 |
Aniline |
199 |
330 |
495 |
632 |
218 |
Source: Adapted from Lindell and Perry (1997a).
Emergency managers typically expect to find hazmat produced, stored, or used at fixed-site facilities such as petrochemical and manufacturing plants. However, such materials are also found in facilities as diverse as warehouses (e.g., agricultural fertilizers and pesticides), water treatment plants (chlorine is used to purify the water), and breweries (ammonia is used as a refrigerant). Hazmat is transported by a variety of modes—ship, barge, pipeline, rail, truck, and air. In general, the quantities of hazmat on ships, barges, and pipelines can be as large as those at many fixed site facilities, but quantities usually are smaller when transported by rail, smaller still when transported by truck, and smallest when transported by air. Small to moderate size releases of less hazardous materials at fixed site facilities are occupational hazards but often pose little risk to public health and safety because the risk area lies within the facility boundary lines. However, releases of this size during hazmat transportation are frequently a public hazard because passers-by can easily enter the risk area and become exposed. The amount that is actually released is often much smaller than the total quantity that is available in the container but prudence dictates that the planning process assume the plausible worst case of complete release within a short period of time (e.g., 10 minutes in the case of toxic gases, see US Environmental Protection Agency, 1987). In addition to the quantity of the hazmat released, the size of the risk area depends upon its chemical and physical properties.
The US DOT groups hazmat into nine different classes—explosives, gases, flammable liquids, flammable solids, oxidizers and organic peroxides, toxic (poisonous) materials and infectious substances, radioactive materials, corrosive materials, and miscellaneous dangerous goods. Each of these hazmat classes is described in the remainder of this section. It is important to be aware that classification of a substance into one of these categories does not mean it cannot be a member of another class. For example, hydrogen sulfide is transported as a compressed gas that is both toxic and flammable.
Explosives
Explosives are chemical compounds or mixtures that undergo a very rapid chemical transformation (faster than the speed of sound) generating a release of large quantities of heat and gas. For example, one volume of nitroglycerin expands to 10,000 volumes when it explodes; it is this rapid increase in volume that creates the surge in pressure characteristic of a blast wave. Explosives vary in their sensitivity to heat and impact. Class A consists of high explosives that detonate (up to 4 mi/sec), producing overpressure, fire, and missile hazards. Class B consists of low explosives that deflagrate (approximately .17 mi/sec—about 4% as fast as a detonation) and cause fires and flying debris (usually referred to as missile hazards). Class C consists of low explosives that are fire hazards only. Explosives can cause casualties and property damage due to overpressure from atmospheric blast waves or missile hazards. Destructive effects from the quantities of explosives found in transportation can be felt as much as a mile or more away from the incident site.
Compressed gases are divided into flammable and nonflammable gases. Nonflammable gases—such as carbon dioxide, helium, and nitrogen—are usually transported in small quantities. These are a significant hazard only if the cylinder valve is broken, causing the contents to escape rapidly through the opening and the container to become a missile hazard. Flammable gases (acetylene, hydrogen, methane) are missile and fire hazards. Rupture of gas containers can launch missiles up to a mile, so evacuation out to this distance is advised if there is a fire. Large quantities of flammable gases, such as railcars of liquefied petroleum gas (LPG), are of significant concern because the released gas will travel downwind after release until it reaches an ignition source such as the pilot light in a water heater or the ignition system in a car. At distances of one-half mile or more, the gas cloud can erupt in a fireball that flashes back toward the release point. Emergency managers need to understand the community-wide hazards associated with fires arising from flammable gases. Consequently, this topic is discussed in greater detail later in this chapter.
Flammable liquids
Flammable liquids, which evolve flammable vapors at 80°F or less, pose a threat similar to flammable gases. A volatile liquid such as gasoline rapidly produces large quantities of vapor that can travel toward an ignition source and erupt in flame when it is reached. When a flammable liquid is spilled on land, there should be a downwind evacuation of at least 300 yards. A flammable liquid that floats downstream on water could be dangerous at even greater distances and one that is toxic requires special consideration (see the section on toxic chemicals, below). A fire involving a flammable liquid should stimulate consideration of an evacuation of 800 yards in all directions.
Flammable solids
Flammable solids self-ignite through friction, absorption of moisture, or spontaneous chemical changes such as residual heat from manufacturing. Flammable solids are somewhat less dangerous than flammable gases or liquids, because they do not disperse over wide areas as gases and liquids do. A large spill requires a downwind evacuation of 100 yards, but a fire should stimulate consideration of an evacuation of 800 yards in all directions.
Oxidizers and organic peroxides
Oxidizers and organic peroxides include halogens (e.g., chlorine and fluorine), peroxides (e.g., hydrogen peroxide and benzoyl peroxide), and hypochlorites. These chemicals destroy metals and organic substances and also enhance the ignition of combustibles (a spill of liquid oxygen can cause the ignition of asphalt roads on a hot summer day). Oxidizers and organic peroxides do not burn, but are hazardous because they promote combustion and some are shock sensitive. A large spill should prompt a downwind evacuation of 500 yards and a fire should initiate an evacuation of 800 yards in all directions.
Toxic chemicals
Toxic chemicals, which can have large impact areas, are classified in a number of ways. DOT Class 2 consists of nonflammable gases and Class 6 is defined as poisons. Class A includes gases and vapors, a small amount of which is an inhalation hazard, whereas Class B consists of liquids or solids that are ingestion or absorption hazards. Many of these chemicals are defined by SARA Title III/EPCRA as EHSs. Toxic materials are a major hazard because of the effects they can produce when inhaled into the lungs, ingested into the stomach by means of contaminated water or food, or absorbed through the skin by direct contact. Of these exposure pathways, inhalation hazard is typically the greatest concern because high concentrations achieved during acute exposure can kill in a matter of seconds. Nonetheless, prolonged ingestion can cause cancers in those who are exposed and also can cause genetic defects in their offspring. Moreover, chemical contamination of victims poses problems for volunteers and professionals providing first aid and transporting victims to hospitals. These chemicals vary substantially in their volatility and toxicity, so evacuation distances following a spill or fire must be determined from the Table of Protective Action Distances in the Emergency Response Guidebook (US Department of Transportation, 2000, see www.dot.gov). Emergency managers need to understand the community-wide hazards that could result from a toxic chemical release. Consequently, this topic is discussed in greater detail later in this chapter.
Infectious substances
Infectious substances have rarely been a significant threat to date because there are relatively few shipments of these substances, they usually are transported in small quantities, and they have restrictive requirements for packaging and marking. However, infectious substances have the potential to be used in terrorist attacks, so emergency managers should knowledgeable about them. This topic is discussed in greater detail in the section on biological hazards.
Radioactive materials
Radioactive materials are substances that undergo spontaneous decay, emitting ionizing radiation in the process. The types and quantities of materials transported in the US generally have very small impact areas. With the exception of nuclear power plants, for which planning is supported by state and federal agencies and electric utilities, releases of radioactive materials are likely to involve small quantities. Nonetheless, even a few grams of a lost radiographic source for industrial or medical X-rays can generate a high level of public concern. Here also, the recently recognized threat of terrorist attack from a “dirty bomb” that uses a conventional explosive to scatter radioactive material over a wide area deserves emergency managers’ attention because of the potential for long-term contamination of central business districts. A large spill should prompt a downwind evacuation of 100 yards and a fire should initiate an evacuation of 300 yards in all directions. Emergency managers need to understand the community-wide hazards that could arise from a release of radioactive materials. Consequently, this topic is discussed in greater detail later in this chapter.
Corrosives
Corrosives, which are substances that destroy living tissue at the point of contact, can be either acidic or alkaline. Examples of acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4), whereas examples of alkaline substances (caustics) include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH4). In addition to producing chemical burns of human and animal tissues, corrosives also degrade metals and plastics. The most frequently used and transported substances in this class are not highly volatile, so the geographical area affected by a spill is likely to be no greater than 100 yards unless the container is involved in a fire or the hazmat enters a waterway (e.g., via storm sewers). These chemicals vary substantially in volatility and toxicity, so evacuation distances following a spill or fire must be determined from the Table of Protective Action Distances in the Emergency Response Guidebook.
Miscellaneous dangerous goods
Miscellaneous dangerous goods, as the name of this category suggests, this class comprises a diverse set of materials such as air bags, certain vegetable oils, polychlorinated biphenyls (PCBs), and white asbestos. Materials in this category are low to moderate fire or health hazards to people within 10-25 yards.
Fires
Flammable materials support rapid oxidization that produces heat and affects biological systems by thermal radiation (burns). As noted earlier in the section on wildfires, combustion requires the three elements of the fire triangle: fuel, which is any substance that will burn; oxygen that will combine with the fuel; and enough heat to ignite the fuel. Combustion usually yields enough heat to sustain the combustion reaction, but it also produces combustion products that might be more dangerous than the heat. Combustion of simple hydrocarbons or alcohols as fuels generally yields carbon dioxide, carbon monoxide, water vapor, and unburned vapors of the fuel as combustion products. More complex and heavier substances such as pesticides also yield carbon dioxide, carbon monoxide, water vapor, and unburned vapors of the fuel. However, they also produce highly toxic chemicals. It can be very difficult to predict what will be the combustion products from a building fire (e.g., an agricultural warehouse) because the temperature of the fire is variable over time and from one location to another in the fire, and the chemicals reacting with each other often are variable over time and from one location to another in the fire.
In understanding combustion, it is important to recognize an important distinction between gases and liquids. A gas is a substance that, at normal temperatures and pressures, will expand to fill the available volume in a space. By contrast, a liquid is a substance that, at normal temperatures and pressures, will spread to cover the available area on a surface. Any liquid contains some molecules that are in a gaseous state; this is called vapor. All liquids generate increasing amounts of vapor as the temperature increases and the pressure decreases. Conversely, at a given temperature and pressure, the amount of vapor in a liquid varies from one substance to another. There are three temperatures of each flammable liquid that are important because they determine the production of vapor. In turn, vapor generation is important because it is the vapor that burns, not the liquid. The three important temperatures of a liquid substance are its boiling point, flash point, and ignition temperature. The boiling point is the temperature of a liquid at which its vapor pressure is equal to atmospheric pressure. Vapor production is negligible when a fuel is below its boiling point but increases significantly once it exceeds this temperature. The flash point of a liquid is the temperature at which it gives off enough vapor to flash momentarily when ignited by a spark or flame. A liquid is defined as combustible if it has a flash point above 100°F. (e.g., kerosene) and flammable if it has a flash point below 100° F. (e.g., gasoline). The final temperature to understand is the ignition temperature, which is the minimum temperature at which a substance becomes so hot that its vapor will ignite even in the absence of an external spark or flame.
Gases and vapors have flammable limits that are defined by the concentration (percent by volume in air) at which ignition can occur in open air or an explosion can occur in a confined space. The lower flammable/explosive limit (LFL/LEL) is the minimum concentration at which ignition will occur. Below that limit the fuel/air mixture is “too lean” to burn. The upper flammable/explosive limit (UFL/UEL) is the maximum concentration at which ignition will occur. Above that limit the fuel/air mixture is “too rich” to burn. When released from a source, a flammable gas or vapor disperses in an approximately circular pattern if there is no wind but in an approximately elliptical pattern in the normal situation in which the wind is blowing (see Figure 5-5).
Figure 5.5: Flammable Plume
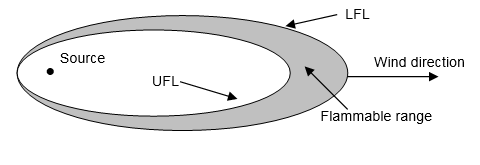
The most dangerous flammable substances have a low ignition temperature, low LEL, and wide flammable range. Indeed, gasoline is widely used precisely because of these characteristics. It has a low flash point (–45 to –36°F), a low LFL (1.4-1.5%), and a reasonably wide range (6%). By contrast, peanut oil is useful in cooking because it has the opposite characteristics—a high flash point (540°F) and an undefined LFL because it does not vaporize.
An important hazard of flammable liquids is a Boiling Liquid Expanding Vapor Explosion (BLEVE), which occurs when a container fails at the same time as the temperature of the contained liquid exceeds its boiling point at normal atmospheric pressure. BLEVEs involve flammable or combustible compressed gases that are not classified as “explosive substances”, but can produce fireballs as large as 1000 feet in diameter and launch shrapnel to distances up to one half mile from the source.
Toxic Industrial Chemical Releases
Toxic industrial chemical releases are of special concern to emergency managers because the airborne dispersion of these chemicals can produce lethal inhalation exposures at distances as great as 10 miles and sometimes even more. The spread of a toxic chemical release can be defined by a dispersion model that includes the hazmat’s chemical and physical characteristics, its release characteristics, the topographic conditions in the release area, and the meteorological conditions at the time of the release. The chemical and physical characteristics of the hazmat include its quantity (measured by the total weight of the hazmat released), volatility (as noted earlier, higher volatility means more chemical becomes airborne per unit of time), buoyancy (whether it tends to flow into low spots because it is heavier than air), and toxicity (the biological effect due to cumulative dose or peak concentration). It also includes the chemical’s physical state—whether it is a solid, liquid (remember, a substance above its boiling point is a vapor), or a gas at ambient temperature and pressure. In general, vapors and gases are major hazards because they are readily inhaled and this is the most rapid path into the body.
Release characteristics are defined by the chemical’s temperature and pressure in relation to ambient conditions, its release rate (in pounds per minute), and the size (surface area) of the spilled pool if the substance is a liquid. Temperature and pressure are important because the rate at which the chemical disperses in the atmosphere increases when these parameters exceed ambient conditions. The release rate is important because it determines the concentration of the chemical in the atmosphere. Specifically, a higher release rate puts a larger volume of chemical into a given volume of air, thus increasing its concentration (where the latter is defined as the volume of chemical divided by the volume of air in which it is located).
Topographical conditions relevant to liquid spills include the slope of the ground and the presence of depressions. As is the case with flooding, steep slopes allow a liquid to rapidly move away from the location of the spill. Both flat slopes and depressions decrease the size of a liquid pool which, in turn, affects the size of the pool’s surface area and reduces the rate at which vapor is generated from it. Thus, dikes are erected around chemical tanks to confine spills in case the tanks leak and hazmat responders build temporary dikes around spills for the same reason. Topographical characteristics also affect the dispersion of a chemical release in the atmosphere. Hills and valleys are land features that channel the wind direction and can increase wind speed at constriction points—for example, where a valley narrows and causes wind speed to increase due to a “funnel” effect. Forests and buildings are rough surfaces that increase turbulence in the wind field, causing greater vertical mixing. By contrast, large water bodies have very smooth surfaces that do not constrain wind direction and, because they provide no wind turbulence, allow a chemical release to maintain a high concentration at ground level where it is most dangerous to people nearby.
The immediate meteorological conditions of concern during a hazmat release are wind speed, wind direction, and atmospheric stability class. The effect of wind speed on atmospheric dispersion can be seen in Figure 5-6, which shows a release dispersing uniformly in all directions when there is no wind (Panel A). Thus, the plume isopleth (contour of constant chemical concentration) corresponding to the Level of Concern (LOC) for this chemical is a circle. The nearby town lies outside the vulnerable zone so its inhabitants would not need to take protective action. However, Panel B describes the situation in which there is a strong wind, so the plume isopleth corresponding to the LOC for this chemical takes the shape of an ellipse. In this case, the nearby town lies inside the vulnerable zone and would need to take protective action.
Figure 5-6. Effects of Wind Speed on Plume Dispersion.
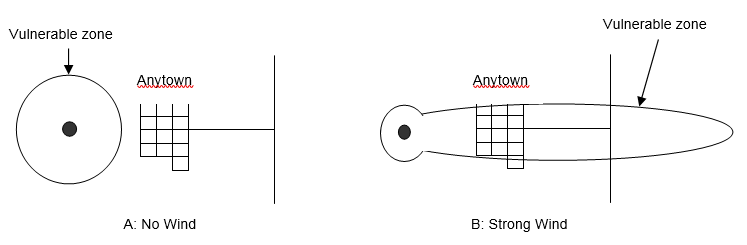
As Table 5-8 indicates, the atmospheric stability class can vary from Class A through Class F. Class A, the most unstable condition, occurs during strong sunlight (e.g., midday) and light wind. This dilutes the released chemical by mixing it into a larger volume of air. Class F identifies the most stable atmospheric conditions, which take place during clear nighttime hours when there is a light wind. These conditions have very little vertical mixing, so the released chemical remains highly concentrated at ground level.
Table 5-8. Atmospheric Stability Classes.
|
Strength of sunlight |
Nighttime conditions |
||||
|
Surface Wind Speed (mph) |
Strong |
Moderate |
Slight |
Overcast ³ 50% |
Overcast < 50% |
|
< 4.5 |
A |
A-B |
B |
- |
- |
|
4.5-6.7 |
A-B |
B |
C |
E |
F |
|
6.7-11.2 |
B |
B-C |
C |
D |
E |
|
11.2-13.4 |
C |
C-D |
D |
D |
D |
|
>13.4 |
C |
D |
D |
D |
D |
A: Extremely Unstable Conditions
B: Moderately Unstable Conditions
C: Slightly Unstable Conditions
D: Neutral Conditions (heavy overcast day or night)
E: Slightly Stable Conditions
F: Moderately Stable Conditions
Source: Adapted from FEMA, DOT, EPA (no date, a).
It is important to recognize that meteorological characteristics can sometimes remain stable for days at a time, but at other times can change from one hour to the next. Figure 5-7, adapted from McKenna (2000), displays the wind direction at each hour during the day of the accident at the Three Mile Island (TMI) nuclear power plant in terms of the orientation of an arrow. Wind speed is indicated by the length of the arrow. The figure shows wind speed and direction changed repeatedly during the course of the accident, so any recommendation to evacuate the area downwind from the plant would have referred to different geographic areas at different times during the day. This would have made evacuation recommendations extremely problematic because the time required to evacuate these areas would have taken many hours. Consequently, the evacuation of one area would have still been in progress when the order to initiate an evacuation in a very different direction was initiated.
Figure 5-7. Wind Rose from 3:00 a.m. to 6:00 p.m. on the First Day of the TMI Accident.
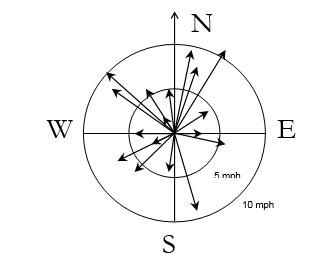
Source: Adapted from McKenna (2000).
The ultimate concern in emergency management is the protection of the population at risk. The risk to this target population varies inversely with distance from the source of the release. Specifically, the concentration (C) of a hazardous material decreases with distance (d) according to the inverse square law (i.e., C = 1/d2). However, distance is not the only factor that should be of concern. In addition, the density of the population should be considered because a greater number of persons per unit area increases risk area population. Moreover, there might be differences in susceptibility within the risk area population because individuals differ in their dose-response relationships as a function of age (the youngest and oldest tend to be the most susceptible) and physical condition (those with compromised immune systems are the most susceptible).
Toxic chemicals differ in their exposure pathways—inhalation, ingestion, and absorption. Inhalation is the means by which entry into the lungs is achieved. This is generally a major concern because toxic materials can pass rapidly through lungs to bloodstream and on to specific organs within minutes of the time that exposure begins. Ingestion is of less immediate concern because entry through the mouth into the digestive system (stomach and intestines) is a slower route into the bloodstream and on to specific organs. Depending on the chemical’s concentration and toxicity, ingestion exposures might be able to be tolerated for days or months. Authorities might choose to prevent ingestion exposures by withholding contaminated food from the market or recommending that those in the risk area drink boiled or bottled water. Absorption involves entry directly through the pores of the skin (or through the eyes), so it is more likely to be a concern for first responders than for local residents. Nonetheless, some chemicals can affect local populations in this way, as was the case with the release of methyl isocyanate during the accident in Bhopal, India, in 1984.
The harmful effects of toxic chemicals are caused by alteration of cellular functions (cell damage or death), which can be either acute or chronic in nature. Acute effects occur during the time period from 0–48 hours. Irritants cause chemical burns (dehydration and exothermic reactions with cell tissue). Asphyxiants are of two types; simple asphyxiants such as carbon dioxide (CO2) displace oxygen (O2) within a confined space or are heavier than oxygen so they displace it in low-lying areas such as ditches. By contrast, chemical asphyxiants prevent the body from using the oxygen even if it is available in the atmosphere. For example, carbon monoxide (CO) combines with the hemoglobin in red blood cells more readily than does O2 so the CO prevents the body from obtaining the available O2 in the air. Anesthetics/narcotics depress the central nervous system and, in extreme cases, suppress autonomic responses such as breathing and heart function.
Chronic, or long-term, effects can be general cell toxins, known as cytotoxins, or have organ specific toxic effects. In the latter case, the word toxin is preceded by a prefix referring to the specific system affected. Consequently, toxins affecting the circulatory system are called hemotoxins, those affecting the liver are hepatotoxins, those affecting the kidneys are nephrotoxins, and those affecting the nervous system referred to as neurotoxins. Other chronic effects of toxic chemicals are to cause cancers, so these chemicals are referred to as carcinogens. Mutagens cause mutations in those directly exposed, whereas teratogens cause mutations to the genetic material of those directly exposed and, thus, mutations in their offspring. The severity of any toxic effect is generally due to a chemical’s rate and extent of absorption into the bloodstream, its rate and extent of transformation into breakdown products, and its rate and extent of excretion of the chemical and its breakdown products from the body (i.e., the substances into which the chemical decomposes).
Research on toxic chemicals has led to the development of dose limits. Some important concepts in defining dose limits are the LD-50, which is the dose (usually of a liquid or solid) that is lethal to half of those exposed, and the LC-50, which is the concentration (usually of a gas) that is lethal to half of those exposed. Based upon these dose levels, authoritative sources have devised dose limits that are administrative quantities that should not be exceeded. LOCs are values provided by EPA indicating the Level of Concern or “concentration of an EHS [Extremely Hazardous Substance] above which there may be serious irreversible health effects or death as a result of a single exposure for a relatively short period of time” (US Environmental Protection Agency, 1987, p. XX). IDLHs are values provided by NIOSH/OSHA indicating the concentration of a gas that is Immediately Dangerous to Life or Health for those exposed more than 30 minutes. TLVs are Threshold Limit Values, which are the amounts that the American Conference of Government Industrial Hygienists has determined that a healthy person can be exposed to 8–10 hours/day, 5 days/week throughout the work life without adverse effects.
Weaponized Toxic Chemicals
Although it seems plausible that a deliberate attack might use explosives to cause release toxic chemicals from a domestic source such as a chemical plant, rail car, or tank truck, it also is possible that a weaponized toxic agent might be used. Such agents were originally used by the military in battles dating back to World War I. Over the years, attention turned to increasingly toxic chemicals that, by their very nature, require smaller doses to achieve a significant effect (e.g., disability or death). One consequence of the more advanced toxic agents is that they can affect victims through absorption in secondary contamination. That is, chemical residues on a victim’s skin or clothing can affect those who handle that individual. Indeed, any object on which the chemical is deposited becomes an avenue of secondary contamination (World Health Organization, 2004). A list of the most likely weaponized toxic agents is presented in Table 5-9. Some of these agents are produced by biological processes (botulism, anthrax, and encephalitis) that affect victims through the production of toxins and, thus, are more properly considered to be chemical weapons (World Health Organization, 2004).
Table 5-9. Weaponized Toxic Agents.
|
Agent |
Example |
|
Tear gases/other sensory irritants |
Oleoresin capsicum (“pepper spray”) |
|
Choking agents (lung irritants) |
Phosgene |
|
Blood gases |
Hydrogen cyanide |
|
Vesicants (blister gases) |
Mustard gas |
|
Nerve gases |
O-Isopropyl Methylphosphonofluoridate (Sarin gas) |
|
Toxins |
Clostrinium botulinum (“botulism”) |
|
Bacteria and rickettsiae |
Bacillus anthracis (“anthrax”) |
|
Viruses |
Equine encephalitis |
Source: Adapted from World Health Organization (2004)
A terrorist attack involving a toxic chemical agent might be detected initially by fire, police, or emergency medical services personnel responding to a report of a mass casualty incident. Likely symptoms include headache, nausea, breathing difficulty, convulsions, or sudden death—especially when these symptoms are displayed by a large number of people in the same place at the same time. In this case, the appropriate response will be the same as in any other hazardous materials incident. Specifically, there will be a need to control access to the incident site, decontaminate the victims as needed, and transport them for definitive medical care. In addition to the normal coordination with emergency medical services and hospital personnel, it is appropriate for emergency managers to be aware of the assistance that is available from local poison control centers. Other than that, the capabilities needed to respond effectively to an attack using toxic chemicals will be much the same as those needed for an industrial accident involving these materials (World Health Organization, 2004). Unfortunately, few communities—even those with a significant number of chemical facilities—have hospitals with the capability to handle mass casualties from toxic chemical exposure caused by either an industrial accident or a terrorist attack.
In the event of a terrorist attack, emergency managers will need to deal with a consideration that is not encountered in most other incidents to which they respond. Specifically, the incident site will be considered a crime scene by law enforcement authorities. Consequently, emergency mangers must learn about the basic procedures these personnel will follow, including collecting evidence, maintaining a chain of custody over that evidence, and controlling access to the incident scene. This latter issue should be carefully coordinated to avoid a conflict between emergency management procedures for victim rescue and law enforcement procedures for crime scene security.
Radiological Material Releases
There are 123 nuclear power plants in the US, most of which are located in Northeast, Southeast, and Midwest. To understand the radiological hazards of these plants, it is necessary to understand the atomic fission reaction. The atoms of chemical elements consist of positively charged protons and neutrally charged neutrons in the atom’s nucleus, together with negatively charged electrons orbiting around the nucleus. Some unstable chemical elements undergo a process of spontaneous decay in which a single atom divides into two less massive atoms (known as fission products) while emitting energy in the form of heat and ionizing radiation. The ionizing radiation can take the form of alpha, beta, or gamma radiation. Alpha radiation can travel only a very short distance and is easily blocked by a sheet of paper but is dangerous when inhaled (e.g., Pu-plutonium). Beta radiation can travel a moderate distance but be blocked by a sheet of aluminum foil. Gamma radiation can travel a long distance and can be blocked only by very dense substances such as stone, concrete, and lead.
Radioactive materials are used for a variety of purposes. Small quantities of some materials are used as sources of radiation for medical and industrial diagnostic purposes (e.g., imaging fractured bones and faulty welds). Large quantities of other radiological materials are used as sources of heat to produce the steam needed to drive electric generators at power plants. In these nuclear power plants, enriched uranium fuel fissions when struck by a free neutron (see Figure 5-8). The thermal energy released is used to heat water and, thus, produce steam. The free neutrons are used to continue a sustained chain reaction and the fission products are waste products that must eventually be disposed in a permanent repository.
The fuel temperature is controlled by cooling water and the reaction rate is controlled by neutron absorbing rods. The amount of fission products increases with age, so the reactor is refueled by moving the fuel in stages toward the center of the reactor vessel. Spent fuel, which contains a significant amount of radioactive fission products and some uranium, is stored onsite until transfer to a repository. The nuclear fuel is located in the plant’s reactor coolant system (RCS), where it is contained in fuel pins that are welded shut and inserted into long rods that are integrated into assemblies. Cooling water is pumped into the reactor vessel where it circulates, picks up heat (and small amounts of radioactive fission products) from the fuel, and flows out of the reactor vessel.
Figure 5-8. The Atomic Fission Reaction.
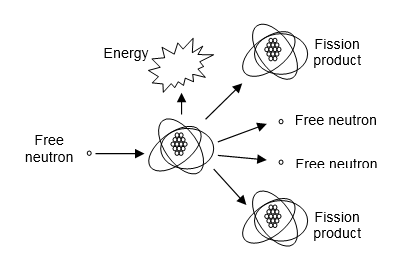
There are two types of RCSs, Pressurized Water Reactors (PWRs) and Boiling Water Reactors (BWRs). In PWRs, the core coolant water is pressurized (the pressurizer is a device used to control the pressure in the reactor vessel) to prevent it from boiling. The hot water passes through a heat exchanger (called the steam generator), gives up its heat, and returns to the reactor vessel, completing the primary coolant system. The water in the secondary coolant system is allowed to boil, producing steam in the steam generator. In BWRs, the core coolant water is allowed to boil, generating steam directly. The steam is delivered to the turbine, spinning it to make electricity. The RCS is located in a containment building (the turbine is in an adjacent building), which is constructed with thick walls of steel-reinforced concrete to withstand high internal pressures or external missile impact. However, it has many penetrations for water pipes, steam pipes and instrumentation and control cables. These penetrations are sealed during normal operations, but the seals could be damaged during an accident that allows radioactive material to escape from the containment building into the environment.
During a severe accident involving irreversible loss of coolant, the fuel will first melt through the steel cladding, then melt through the RCS, and finally escape the containment building (probably through a basemat melt-through or steam explosion). This process could produce a release as soon as 45-90 minutes after core uncovery. If the core melts, the danger to offsite locations depends upon containment integrity. Early health effects are likely if there is early total containment failure and are possible if there is early major containment leakage. Otherwise, early health effects are unlikely. The problem is that containment failure might not be predictable (McKenna, 2001).
A radioactive release would involve a mix of radionuclides (i.e., a variety of radioactive substances that vary in their atomic weight) and this mix is called the source term. The source term is defined by three classes of radionuclides—particulates, radioiodine, and noble gases. Particulates include uranium (U) and strontium (Sr), the latter of which is dangerous because it is chemically similar to calcium (Ca) and therefore tends to be deposited in bone marrow. Radioiodine (I-131) is dangerous because it substitutes for nonradioactive iodine in the thyroid and, thus, can cause thyroid cancer. Noble gases such as krypton (Kr) do not react chemically with anything, but are easily inhaled to produce radiation exposures while they remain in the lungs.
The source term is also characterized by its volatility. As noted in connection with toxic chemicals, volatility is an important characteristic of a substance because higher volatility means more of the radionuclide becomes airborne per unit of time and stays airborne. The quantity of radioactivity released can be measured in terms of the number of pounds or kilograms, but this is not a very useful measure because two different source terms with the same mass might emit very different levels of radiation. Consequently, the amount of radioactivity (ionizing radiation) released is measured by the number of disintegrations per unit time (Curies). In fact, these disintegrations are what a Geiger counter measures. The amount of radioactivity is usually measured in curies of an individual radionuclide or class of radionuclides.
Exposure pathways for radiological materials are similar to those of toxic chemicals. Breathing air that is contaminated with radioactive materials can cause inhalation exposure and eating food (e.g., unwashed local produce) or drinking liquids (e.g., water or milk) that is contaminated can cause ingestion exposure. Contamination also can enter the body through an open wound such as a compound fracture, laceration, or abrasion, but radiological materials do not cause absorption exposures because they do not pass through the skin. However, because radiological materials release energy, they can produce exposures via direct radiation (also known as “shine”) from a plume that is passing overhead. If the plume has a significant component of particulates, these might be deposited on the ground, vegetation, vehicles, or buildings and the direct radiation from the deposited particulates would produce a continuing exposure. In some cases, the small particles of deposited material could become resuspended and inhaled or ingested. In this connection, it is important to recognize the distinction between irradiation and contamination. Irradiation involves the transmission of energy to a target that absorbs it, whereas contamination occurs when radioactive particles are deposited in a location within the body where they provide continuing irradiation.
Measuring radiation dose is somewhat more complicated than measuring doses of toxic chemicals. As noted earlier, a Curie is a measure of the activity of a radioactive source in atomic disintegrations/second, whereas a Roentgen is a measure of exposure to ionizing radiation. A rad is a measure of absorbed dose, and a rem (“Roentgen equivalent man”) is a measure of committed dose equivalent. The term committed refers to the fact that contamination by radioactive material on the skin or absorbed into the body will continue to administer a dose until it decays or is removed. The term equivalent refers to the fact that there are differences in the biological effects of alpha, beta, and gamma radiation. Weighting factors are used to make adjustments for the biological effects of the different types of radiation. However, for offsite emergency planning purposes, one rem is approximately equal to one rad.
The health effects of exposure to ionizing radiation are defined as early fatalities, prodromal effects, and delayed effects. Early fatalities occur within a period of days or weeks and are readily interpreted as effects of radiation exposure. Early fatalities begin to appear at whole body absorbed doses of 140 rad (which is equal to 1.4 Gray, the new international scientific unit) but less than 5% of the population would be expected to die from such exposures. Approximately 50% of an exposed population would be expected to die from a whole body dose of 300 rad and 95% would be expected to die from a dose of 460 rad.
Prodromal effects are early symptoms of more serious health effects (e.g., abnormal skin redness, loss of appetite, nausea, diarrhea, nonmalignant skin damage), whereas delayed effects are cancers that might take decades to manifest themselves and might only be associated with a particular exposure on a statistical basis. Genetic disorders do not reveal their effects until the next generation is born. Prodromal effects would be expected to manifest themselves in less than 2% of the population at a dose of 50 rad, whereas 50% would be expected to exhibit prodromal symptoms at 150 rad and 98% would be expected to show these symptoms at 250 rad.
The delayed effects of radiation exposure can be seen in Table 5-10, which lists the number of fatal cancers, nonfatal cancers, and genetic disorders that can be expected as a function of the number of person-rem (that is, the number of persons exposed times the number of rems of exposure per person). The small numbers involved are indicated by the fact that the coefficients are presented in scientific notation (i.e., 2.8 E-4 = .00028). That is, 2.8 fatal cancers, 2.4 nonfatal cancers, and 1 genetic effect are expected if 10,000 people are each exposed to 1 rem of radiation to the whole body.
Table 5-10. Average Risk of Delayed Effects (Per Person-Rem)
| Effect | Whole Body | Thyroid | Skin |
| Fatal Cancers | 2.8 E-4 | 3.6 E-5 | 3.0 E-6 |
| Nonfatal Cancers | 2.4 E-4 | 3.2 E-4 | 3.0 E-4 |
| Genetic Disorders | 1.0E-4 |
It is important to be aware of the differential biological affinity of radionuclides for specific organs. Whole body radiation refers to the response of the “typical” cell to irradiation, reflecting the common components and structures all cells share. By contrast, the thyroid is sensitive to I-131 and bone marrow is sensitive to Sr-90. Organ differences in dose-response arise because rapidly dividing cells, found in the gut (damage causes diarrhea and vomiting) and hair follicles (damage causes hair loss), are especially susceptible. There also are individual differences in dose-response. For example, fetuses are extremely susceptible because all of their cells are dividing rapidly, and the same is generally true of preschool children. Unfortunately, recommendations for protective action by pregnant women are easily misinterpreted. The concern is for the health of the highly susceptible fetus, not that of the much less susceptible adult woman. Other population segments include those at risk of any environmental insult: the very old, the very young, and those with compromised immune systems.
Population protective actions for radiological emergencies are based upon three fundamental attenuation factors—time, distance, and shielding. Evacuation reduces the amount of time exposed and increases distance from the source, whereas sheltering in-place can provide shielding if this is done within dense materials that absorb energy and are airtight. To determine when protective action should be initiated, the EPA has developed Early Phase Protective Action Guides (PAGs), which are specific criteria for initiating population protective action in radiological emergencies (Conklin & Edwards, 2001). Note that the whole body dose listed in Table 5-11 for initiating evacuation (1 rem) is only a small fraction of the exposure level that would be expected to produce prodromal effects in the most susceptible 2% of the general population.
Table 5-11. EPA Protective Action Guides
|
Organ |
EPA PAGsa (rem/Sv) |
Protective Actionb |
|
Whole body |
1-5 (.01-.05) |
Evacuation |
|
Thyroid |
25 (.25) |
Stable Iodine (KI) |
a Dose inhalation from and external exposure from plume and ground deposition.
b Actions should be taken to avert PAG dose.
* Evacuation is considered to be the most effective protective action for nuclear power plant accidents at American sites.
Biological Hazards
According to the World Health Organization (2004, p. 5), biological weapons are “those that achieve their intended target effects through the infectivity of disease-causing micro-organisms and other such entities including viruses, infectious nucleic acids, and prions”. Some biological agents produce toxins and, thus, are actually chemical weapons whose “chemical action on life processes [is] capable of causing death, temporary incapacitation or permanent harm” (World Health Organization, 2004, p. 6).
Emergency managers should recognize that most biological agents likely to be used in deliberate attacks on their communities also exist as natural hazards. They also could be released accidentally from fixed-site facilities (e.g., commercial or academic laboratories) or in transportation among those facilities. These biological agents exist at low levels of prevalence in human populations or, alternatively, in animal populations from which they can spread to human populations. Indeed, one quarter of the world’s deaths in 1998 were caused by infectious diseases. The major consequence of most biological agents is the magnification of their effects by infection, unlike chemical agents that generally experience dissipation over time and distance. Biological agents magnify their effects by multiplying within the target organisms, but chemical agents cannot do this.
Biological agents can be dispersed by contaminating food or water to achieve exposure through ingestion. For example, a terrorist attack might attempt to introduce a plant or animal infection that would affect people through the food distribution system. However, this system is routinely monitored by the US Department of Agriculture and state departments of agriculture. In some cases, these agencies already receive support from state emergency management agencies when natural outbreaks occur. For example, collaborative relationships have been demonstrated in recent cases of Bovine Spongiform Encephalopathy (BSE—“mad cow” disease) and naturally occurring outbreaks of livestock anthrax.
Alternatively, a biological agent can be used to create an aerosol cloud of liquid droplets or solid particles to achieve an inhalation hazard. The aerosol can be dispersed either in the open environment or through a building’s heating, ventilation, and air conditioning (HVAC) system, but the latter is likely to produce more casualties because the concentration of the biological agent will be greater. The effectiveness of the dispersion will depend on the hazard agent’s physical (particle size and weight) characteristics. Micrometeorological variation can produce corresponding variation in the dispersion of the hazard agent and, under certain conditions, extreme dilution or loss of its viability. Nonetheless, epidemic spread could compensate for poor initial dispersion.
As is the case with some toxic chemical agents, biological agents can be very difficult to detect when symptoms do not appear until long after exposure occurs. The incubation period for biological agents is free of symptoms, so tourists or business travelers might travel a long way from the attack site before they become symptomatic. Consequently, infection with a contagious agent could cause secondary outbreaks that are caused by victims of the initial exposure transmitting the agent to people with whom they come into contact during their travels. Thus, infection can spread widely before local authorities are aware that an attack has even occurred.
The dispersal of the victims at the time the symptoms are manifested and the similarity of these symptoms to those of routinely encountered diseases such as influenza could impede prompt recognition of an attack. The major problem here is that the symptoms of biological agents are frequently indistinguishable from common maladies such as colds and influenza. Consequently, the occurrence of a covert biological agent release is most likely to be identified by noting a significant increase in the incidence of such symptoms. This would either be achieved by health care providers in emergency rooms and clinics supplemented by the health surveillance system operated by the public health department.
There is an emerging sensor technology for detecting many biological agents. These sensors can identify the presence of agents at a very early stage rather than awaiting the development of symptoms in human populations. However, they can only detect these agents at specific locations and, because of their expense, cannot currently be widely distributed. For the foreseeable future, their deployment is likely to be limited to the most critical facilities. Consequently, it is important for emergency managers to establish a working relationship with their local health departments. In turn, these will have established contacts with regional laboratories and state and federal public health agencies to provide assistance in identifying the agent, treating the victims, and decontaminating the incident site.
Countermeasures for biological agents include isolation and quarantine. Isolation is the action taken to prevent those who are known to be ill with a contagious disease from infecting others. It typically is associated with special treatment to remedy the disease. By contrast, quarantine is used to prevent those who might have been exposed to a biological agent but do not currently exhibit symptoms. Thus, they might not become ill and, indeed, they might not even have the disease. However, it is critical to prevent them from infecting others. Thus, quarantine is somewhat similar to sheltering in-place from toxic chemical hazards. The difference is that people being quarantined are asked (or legally required) to remain indoors in order to protect others from themselves (because they are the hazard) rather than to protect themselves from an external hazard. Although there is extensive research on household compliance with evacuation warnings, the same cannot be said for isolation and quarantine. Nonetheless, it seems safe to say the level of compliance will be less than perfect, so emergency managers should try to assess local residents’ perceptions of these protective actions if the need to implement them arises.
In addition, biological agents can be combated by vaccines that provide protection against specific agents and other therapeutic agents that seek to block the body’s reaction to the agent. Emergency managers will be particularly interested in the latter type of therapy because a generic therapeutic mechanism would be effective against a wide variety of biological agents, just as a wide-spectrum antibiotic is effective against a range of bacteria.

